PLOS Neglected Tropical Diseases: The Art of Writing and Implementing Standard Operating Procedures (SOPs) for Laboratories in Low-Resource Settings: Review of Guidelines and Best Practices

Fillable Online erasmus ankara edu 1 COOPERATIONPROJECT INFORMATION Discipline Academic field (ISCED 2013) 0421 Law 0488 Business, administration and law, interdisciplinary programmes - erasmus ankara edu Fax Email Print - pdfFiller

ISO 15223-1:2021(en), Medical devices — Symbols to be used with information to be supplied by the manufacturer — Part 1: General requirements
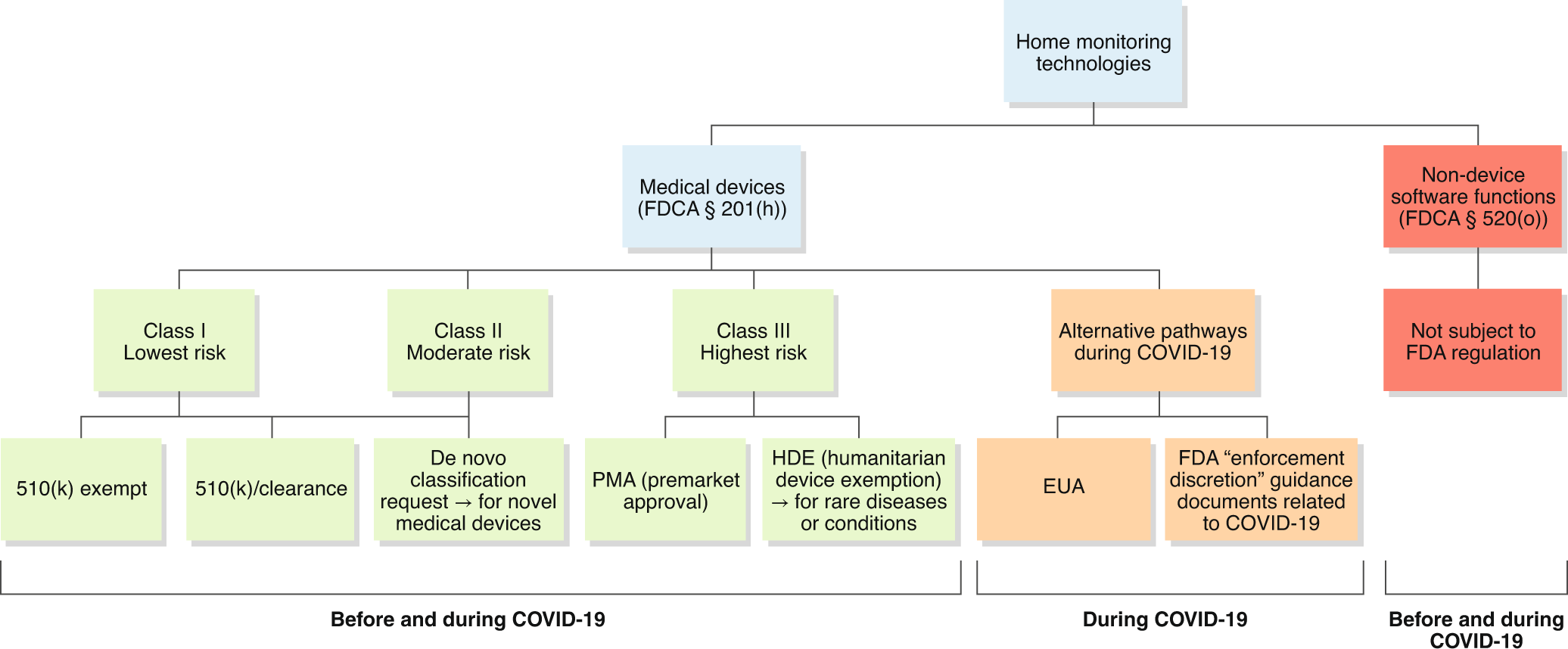
Regulatory, safety, and privacy concerns of home monitoring technologies during COVID-19 | Nature Medicine

Inhaled isoflurane via the anaesthetic conserving device versus propofol for sedation of invasively ventilated patients in intensive care units in Germany and Slovenia: an open-label, phase 3, randomised controlled, non-inferiority trial -