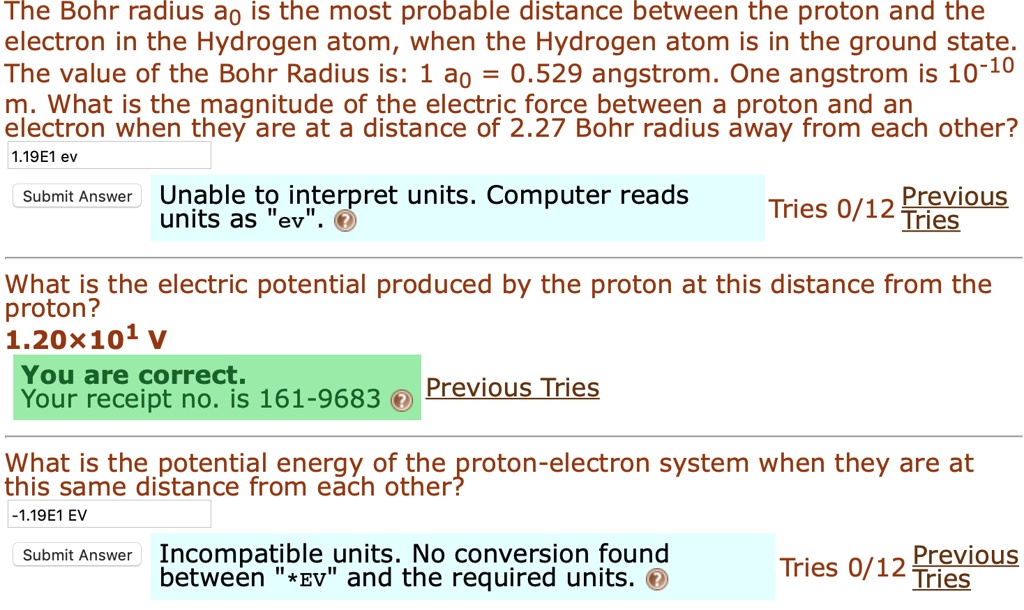
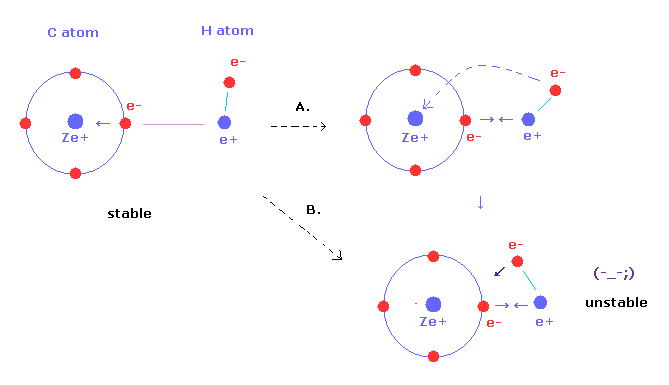
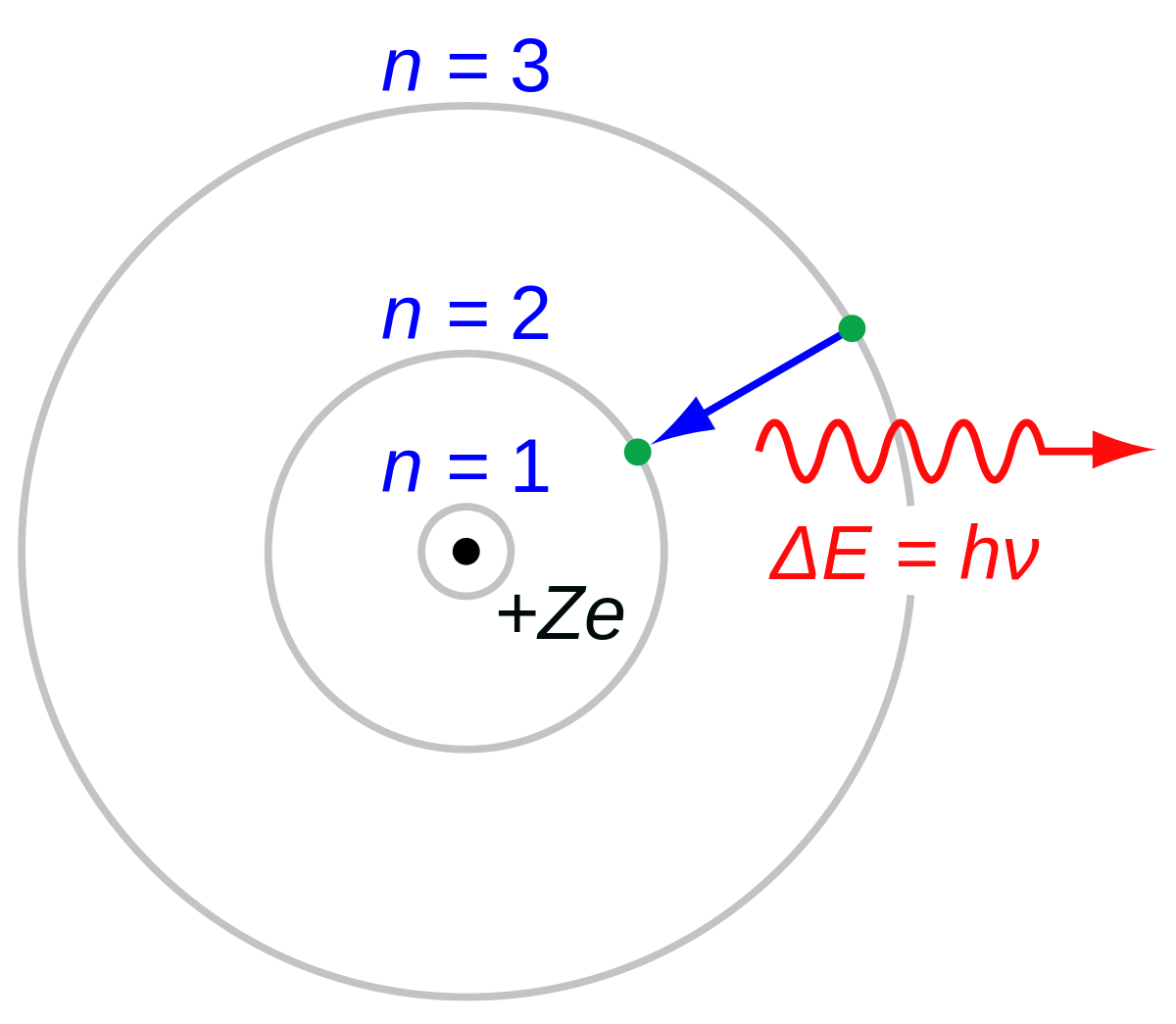
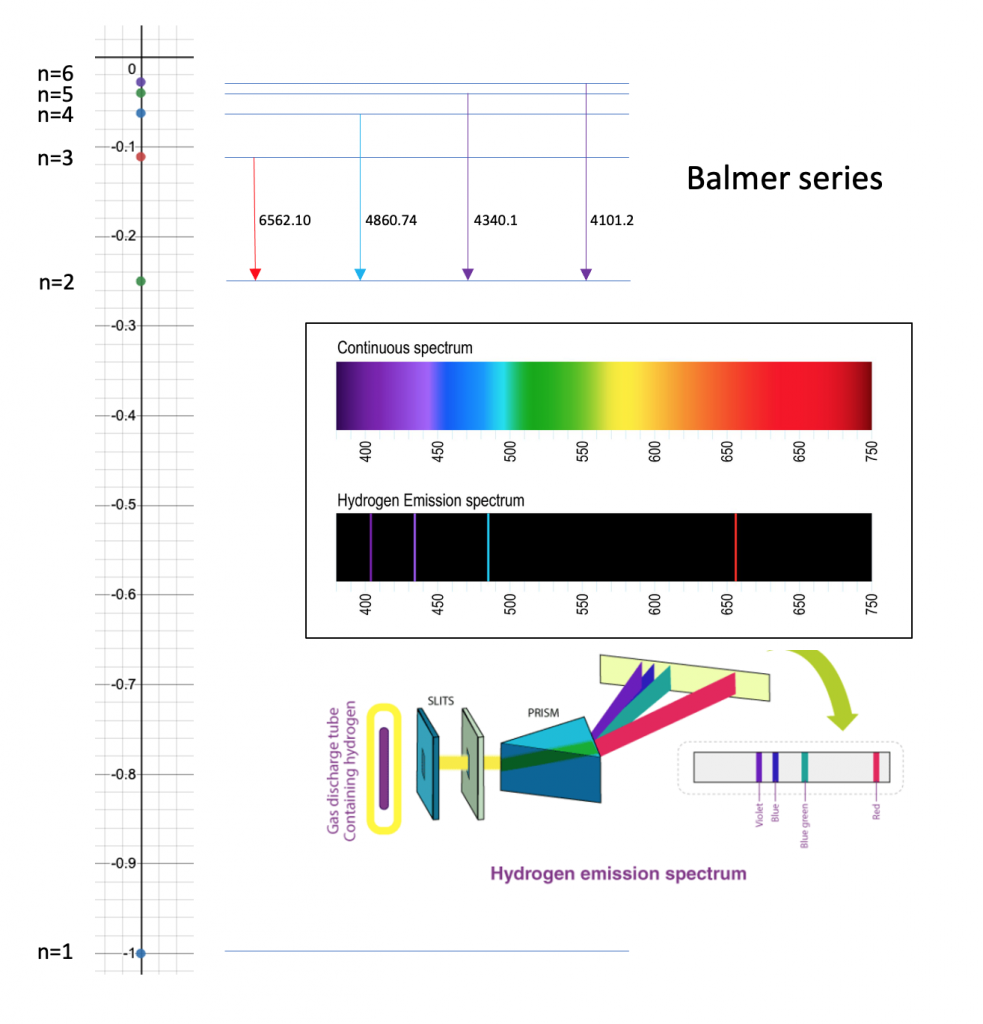
Bohr radius for hydrogen atom ( n=1) is approximately 0.530 angstroms. What is th radius for first excited - Brainly.in

Calculate the wavelength in Angstroms of the photon that is emitted when an electron is Bohr orb... - YouTube
WARNING in particle_methods.F:684 :: The distance between the atoms *** *** 10 and 405 is only 0.496 angstrom and thus smaller than the threshold *** *** of 0.500 angstrom
Bohr Model of the Atom. Experimental Observation of Hydrogen Line Emission In 1853, Anders Angstrom of Sweden first determined that a set of discrete. - ppt download

SOLVED:(a) According to the Bohr model, an electron in the ground state of a hydrogen atom orbits the nucleus at a specific radius of 0.53 Å. In the quantum mechanical description of

Calculate the wavelength in Angstroms of the photon that is emitted when an electron is Bohr orbit n = 2 return to the orbit n = 1 in the hydrogen atom .The

the radius of first bohr Orbit for hydrogen is 0.53 Amsterdam the radius of third Bohr's orbit will - Brainly.in

Bohr Model of the Atom. Experimental Observation of Hydrogen Line Emission In 1853, Anders Angstrom of Sweden first determined that a set of discrete. - ppt download

Rugwed Lokhande on Twitter: ""Hierarchical Partition of Hilbert Space Bases on Excitation and Seniority weightage" #RSCPoster #RSCphys https://t.co/pSurtTZWvX" / Twitter
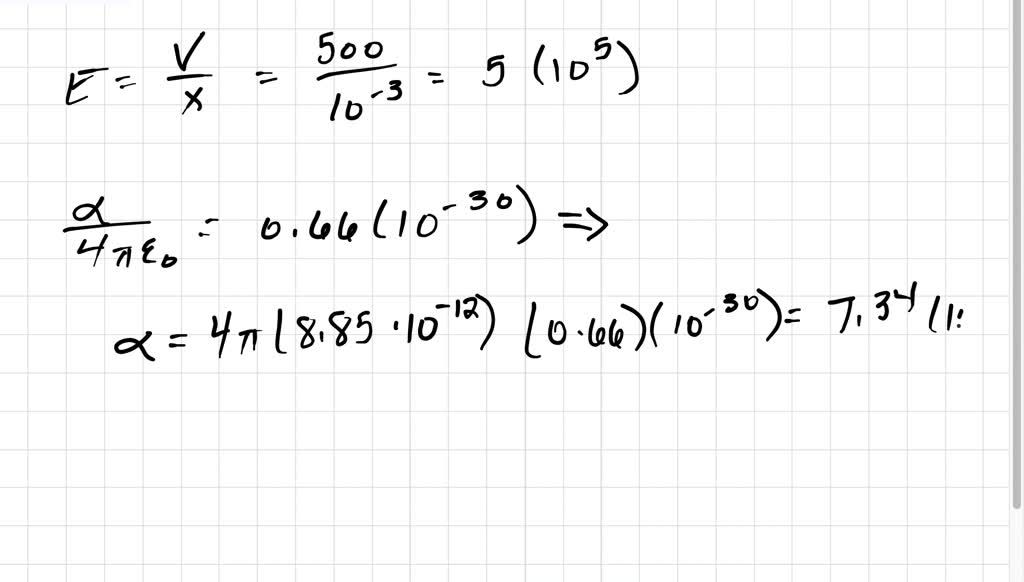
SOLVED:A hydrogen atom (with the Bohr radius of half an angstrom) is situated between two metal plates 1 mm apart, which are connected to opposite terminals of a 500 V battery. What